15 years of St. Gallen OPEX benchmarking – A success story
It was in 2004 when the Institute of Technology Management at the University of St. Gallen, together with some major players in the pharmaceutical industry, launched the first benchmarking study on the topic of “Operational Excellence” (OPEX). Already then, almost one hundred production sites took part. 15 years later, the St. Gallen OPEX benchmarking is an established brand in the industry. On the occasion of the anniversary, the Institute of Technology Management and its director, Professor Dr. Thomas Friedli, initiator of the benchmarking, can look back on successful years.
The St. Gallen approach
The pharmaceutical industry had been a laggard in adopting improvement programs in manufacturing compared to other industries that started adopting lean manufacturing in the 1990s or like the pioneer Toyota already after World War II. Driven by expiring patents and regulators that started paying more attention to effective but as well efficient manufacturing processes to support the access to affordable medicine, pharmaceutical companies saw the comparison among themselves and the exchange of successful practices as an opportunity to turn into continuously improving organizations.
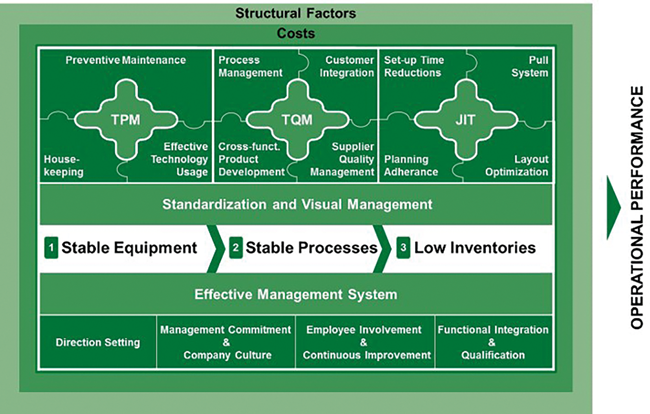
With its location in Switzerland, its proximity to the industry, and its systems theory background, the University of St. Gallen proved to be the ideal partner for such an undertaking. Scientifically influenced by system thinking, the Institute of Technology Management formalized “Operational Excellence” in its St. Gallen OPEX model (fig. 1). OPEX constitutes the balanced management of cost, quality, and time, eventually focusing on customer needs. Contrary to one-dimensional optimization approaches, the St. Gallen OPEX model already then offered a holistic approach, comprising effectiveness and efficiency, and the ideal framework to compare manufacturing plants in a meaningful and comprehensive way. The model’s building blocks, Total Productive Maintenance (TPM), Total Quality Management (TQM), Just-in-Time (JIT), and the underlying management system ensure a distinct and logical structure in which performance metrics are allocated. However, not only is OPEX concerned with performance itself but also with practices (enablers) that lead to a continuously improving organization. Thus, from the very start on, manufacturing plants have also been compared regarding their adoption of such practices, eventually allowing them to derive improvement potentials in different categories. Eventually, both performance metrics and enablers cannot be evaluated in isolation but need to be assessed in consideration of structural factors and the site’s environment.
An established brand for the industry and regulatory agencies
Continuous improvement is not only the goal of participating companies, but it also applies to the St. Gallen benchmarking itself. Since 2008, it is possible for pharmaceutical companies to conduct a St. Gallen benchmarking and to position themselves against the existing database of manufacturing sites at any time. Five years ago, the benchmarking has been revised and extended to allow for a more in-depth analysis as well as a more meaningful comparison among plants manufacturing different drug substances and products. The latest update constituted the inclusion of a full operationalization of the ICH Q10 guideline. It is no surprise, therefore, that the St. Gallen OPEX benchmarking is still in demand as it was then. Throughout the years, the institute accumulated the largest independent database for pharmaceutical production consisting of almost 400 manufacturing sites scattered around the globe – and it is still growing.
Not only does this unique database catch attention from pharmaceutical companies but also regulatory agencies. In 2016, the U.S. Food and Drug Administration (FDA) awarded a research grant, linked to FDA’s Pharmaceutical Manufacturing Quality Metrics Initiative, to the University of St. Gallen in order to provide a scientific base to the FDA. The results were compiled in three comprehensive reports, each covering one year of dedicated research.
This year the Institute of Technology Management teamed up with Dun & Bradstreet to conduct a study funded by the FDA. The 2020 Quality Benchmarking Study is a global initiative that will create a baseline of the state of pharmaceutical manufacturing quality management practices and is aiming for two thousand participating manufacturing sites from 52 countries. Similar to the Quality Metrics Research project, the St. Gallen OPEX benchmarking database had been the scientific basis for this study to be set up. A sub-set of metrics as well as enablers was thoroughly derived that most precisely reflect the holistic approach of the St. Gallen understanding of OPEX. The outcome of this study will reveal insights into the current status of quality management maturity and its link to production efficiency.
15 years anniversary event
The St. Gallen OPEX benchmarking has been a success story ever since. For the 15th anniversary, the Institute of Technology Management will host a one-day event on the development and the current state of OPEX in the pharmaceutical industry (fig. 2). By doing so, Professor Friedli with his institute will look back on eventful years. The most insightful research findings from FDA projects will be presented. Partners from the industry will share their experience on their way to OPEX. This setting will be ideal for reflecting upon the progress that was already made and what is still necessary to become world-class in pharmaceutical manufacturing.

Celebrate with us and join us in Berlin at the Steigenberger Hotel Berlin on November 25 (https://item.unisg.ch/berlin)!
Further information:
Institute of Technology Management
Dufourstrasse 40a
9000 St. Gallen (Switzerland)
Tel.: +41 (0) 712247272
e-mail: mark.grothkopp@unisg.ch